Introduction
|
|
An overview of HIV infection
It has been estimated that the HIV infections has affected around 40 million people worldwide, while about 25 million have died already [1]. Almost two-thirds of the HIV infected population lives in Africa and it causes more deaths than any other disease including malaria [2]. In Kenya, the situation has improved. According to the Kenya Demographic and Health Survey (KDHS) there has been a decline in the sero prevalence of HIV by 15%. Despite the decline in prevalence, HIV still remains one of the most important public health problem faced by the country. One of the consequences of the epidemic is an increase in the number of orphans. It has been estimated that there are currently 100,000 children living with HIV in Kenya and majority have acquired the infection through maternal child transmission. Without appropriate diagnosis and treatment more than half of all the HIV infected children die in the first three years of life [3]. It is, therefore, crucial to diagnose HIV infection early to be able to introduce life prolonging intervention [4]. The diagnosis of HIV infection in children is difficult if based on the signs and symptoms alone since these often overlap with the symptoms seen in malnutrition and other tropical diseases. Hence laboratory investigations involving detection of HIV antibodies in blood are required [5]. The World Health Organization (WHO) has devised clinical parameters to stage the HIV disease in children that can be used as a guide for treatment initiation [6]. In addition, a revised Centre for Disease control (CDC) classification for pediatric HIV infection assesses the severity of HIV infection according to three parameters namely infection status, clinical status and immunological status [5].
Oral health status of HIV infected children
Oral health has been defined as the absence of disease and optimal functioning of the mouth and its tissues in a manner that preserves the highest level of self-esteem; as good oral health is an essential and important component and it is a birthright of every individual in the world' WHO [5]. The principles of good oral health care are the same for patients who are HIV positive as well as the patients without HIV infection though presence of soft tissue and periodontal disease may require more frequent evaluation in pediatric HIV infected children. Oral lesions associated with HIV infection are painful and may present cosmetic problems. With the advent of better methods of detection and improved therapies, HIV infected children are surviving longer and thus coming under the care of a host of affiliated medical personnel including dentists [7]. Generally, successful management improves the quality of life of these children [8].
Ramos et al. reported high plaque prevalence in US children infected with HIV which was significantly associated with age and more common in children with candidiasis. Gelbier et al. examined children in the UK 6 months to 18 years and noted visible plaque deposits in half the children and mean plaque scores of 16.7 and 8.0 in the primary and permanent teeth. Locally, Masiga et al. found the prevalence of gingivitis to have been 37% in normal pre-school children. Gelbier et al. found gingivitis was present in 40% of the children. The mean score was 5.1 and 5.7 in primary and permanent dentitions respectively. Ramos et al. noted that the prevalence of gingivitis was significantly associated with age similar to that of plaque and the presence of gingivitis at age 0 year was 6%, one year was 55%, two years was 85%, three years was 87%, four years was 66% and was more strongly associated with the number of teeth. Okunseri et al. reported that the association between conventional gingivitis and low cluster of differentiation 4 (CD4) count was significant p = 0.001. Riberio et al. reported that 58.9% (33 out of 56) of the HIV infected Brazilian children aged between 0 to 14 years in their study presented with gingivitis. [8]
[9] [10] [11] [12] [13]
Age in relation to cluster of differentiation 4 (CD4) count and immunity
The Centers for Diseases Control [13] developed a protocol and categorized the immune status of the children based on the CD4 counts and the CD4 count percent and by age where; one was not immune depressed, two moderately immune depressed while three were severely immune depressed. Each age group was further identified by the CD4 count and the CD4 percent for each diagnosed child, for example, children who were allocated one meaning not immune suppressed were aged =12 months had a CD4 count of =1500 and a CD4% of 25, 1–5 year(s) had a CD4 = 1000 and a CD4% of 25 while the 6–12 years old had and CD4 count of ≥600 and CD4% of 25. In the second category which was moderately immunosuppressed the children aged ≤12 months had a CD4 count of was between 750–1499 while the CD4% was between 15–24, 1–5 year had a CD4 count of 600–999 and a CD4% of 15–24 while the 6–12 years old had and CD4 count of 200–499 and CD4% of CD4% of 15–24. The third which was severely immunosuppressed the respective age group CD4 count and CD4% was ≤12 months ≤750 and ≤15%, 1–5 year had a CD4 count of ≤500 and a CD4% of ≤15 and 6–12 years old CD4 count of ≤200 and CD4% of ≤15.
|
Materials and Methods
|
|
The study involved HIV infected children from Children's homes namely Nyumbani Children's Home which is a refuge for HIV/AIDS-affected, abandoned or orphaned children from age's newborn to 23 years. The children receive comprehensive medical, nutritional, dental, life-skills, psychological, academic and spiritual care as they live in this surrogate family. New Life Children's Home takes in abandoned and orphaned HIV infected babies. The home nurtures them into health and provides for children's spiritual and emotional needs while identifying adoption families.
Nyumbani Children's Homes and New Life children's homes are two of the most established care centers for HIV infected children in Kenya and they were among the first institutions to administer ARV therapy to children and also have well trained medical staff to attend to the children.
The other two sites were outpatient Comprehensive Care Centers at the Kenyatta National Hospital (KNH) and the Coast Province General Hospital (CPGH). The outpatient institutions provided a family environment which provided a wholesome life and enabled the children to cope with the disease.
Kenyatta National Hospital is a referral hospital for the country and it runs a comprehensive care center for HIV positive patients both adults and children and is one of the referral centers in Nairobi. The hospital initiated the protocol for the care and management of HIV care in pediatric patients which was commenced in 2003 [14].The clinic have a free baseline investigations and therapy for the children.
The Coast Province General Hospital is one of the largest provincial hospitals in Kenya and it has a comprehensive care clinic where HIV infected children are reviewed and managed. These children are enrolled into the clinic through the outpatient facilities, referrals from various clinics, nursing homes, district hospitals and children's homes across the province.
This was a descriptive cross-sectional study carried out over a period of three months to assess the oral health status of children living in surrogate families in adoption institutions and those attending the outpatient clinics but living with the natural families.
Sampling and sample size
The total calculated sample size was 228 children from four institutions based on the formula: n = Z2XP (1-P)/d2 at a confidence interval CI 95% with prevalence of gingivitis at 17.5% and dental plaque at 5% and the standard error 1.96. All children who were present at the homes and satisfied the inclusion criteria were examined. The rest of the study population consisted of those who attended the comprehensive care clinics and the two public outpatient clinics and convenience sampling method was used to obtain the calculated sample size of 228, however, 237 children were examined.
Age groups
The 237 children aged 2–15 years were categorized by age and the immune status as was reported by the attending doctor based on the CDC revised classification for pediatric HIV infection [6].
Instruments for data collection
A specially structured questionnaire was used to assess the oral hygiene practices of the child, any oral complaints and whether child was on ARV therapy. The questionnaire was filled by the child's care giver while the child was being examined. The data collection form was a modified WHO oral health assessment form [15] [16] on which the demographic characteristics of the study population and specific oral health status components were recorded. These included the plaque scores, gingival scores, dental caries status and specific HIV related oral manifestations. The Federation Dentaire Internationale (FDI) [17] dental annotation recording system was used for identification of the teeth.
Clinical examination
This was conducted with the patients either seated on a chair or supine on an examination table under natural light using mouth mirrors, periodontal probes, explorers and spatulas for retraction in accordance with the WHO criteria for field studies [15]. In case, a child presented in the mixed dentition stage then the succedaneous tooth was examined or the adjacent tooth was examined.
Oral hygiene
A total of 237 children and adolescents infected and living with HIV aged 2–15 years were examined for plague. Plaque disclosing tablets were used to assess the plaque scores on the index teeth. The WHO plaque index 1997 was used [16]. Plaque was scored on the buccal and lingual surfaces of specific teeth respectively with a scoring criteria of 0 = no plaque detected; 1 = plaque covering less than one-third of tooth surface; 2 = plaque covering up to two-thirds of the tooth surface and 3 = plaque covering entire tooth surface. The buccal surfaces of the primary dentition used were 51, 55, 65, and 71; while the lingual surfaces 75, 85. For the permanent dentition the buccal surfaces which were examined for plaque were 11, 16, 26, 31 and for the lingual surfaces teeth numbers 36, 46.
|
Results
|
|
Distribution of the children by age and gender
The study population comprised of 112 (47.3%) boys and 125 (52.7%) girls. The children had their ages range between 2–15 years with a mean age of 7.5 years (95%CI 7.1–7.9±3.32), and a modal age of nine years. The children were further divided into age cohorts of 2–5, 6–10, and 11–15 years. This was due do the different stages of development which may at times influence oral health status. There were 76 (32%) children aged 2–5 years, 113 (48%) aged 6–10 years while those aged 11–15 years were only 48 (20%) (Figure 1). However, there were no significant statistical differences noted among the gender distribution according to the various age groups. Chi 0.334; 2df; p = 0.84 at 95% CL.
|
|
|
|
Oral Health Practices
|
|
Frequency of brushing and oral hygiene scores
Among the children examined 202 (85.2%) reported brushing their teeth while 35 (14.8%) did not. Among those who brushed once daily, 21 (19.8%) presented with good OH scores, 65 (61.3%) had a fair OH and 20 (18.9%) poor OH. Among those children who reported brushing twice daily, 2 (2.1%) had good OH, 78 (81.3%) fair OH and 16 (16.7%) poor OH while those who did not brush, 4 (11.4%) presented with good OH while 18 (51.4%) had fair OH scores and 13 (37.1%) poor OH scores (Figure 2). Children who reported brushing twice daily had better oral hygiene than those who brushed once and those who did not and the chi square 3.99; 4df; p=0.000 at 95% CL was statistically significant.
Dental checkup and hygiene scores
Among the children examined 100 (42.2%) reported having visited a dentist while 137 (57.8%) had not. Among those who had, 6 (6%) had good OH, 77 (77%) fair OH and 17 (17%) poor OH. Twenty-one (15.3%) children who had not had a dental checkup had good OH, 84 (61.3%) fair OH and 17 (17%) poor OH scores. Chi square was; 7.639; 2df; p = 0.022 at 95 % CL which statistically significant.
Distribution of oral hygiene scores among the age groups
In the two to five years old age group 12 (15.8%) children presented with good OH, 45 (59.2%) fair OH and 19 (25%) poor OH. Among the six to eleven years old children 10 (8.8%) had good OH, 81 (71.7%) fair OH and 22 (19.5%) poor OH. Only 5 (10.4%) adolescents had good OH scores while 35 (72.9%) had fair OH and 8 (16.7%) poor OH. However, the chi square test did not show any significant changes in the oral hygiene scores among the various age groups chi 4.372, 4df: p = 0.358 at 95% CL. Comparison of gender in the specific age groups could not be determined due to lack of statistical power.
Oral hygiene status in the study population
In this study only 27 children (11.4%) had a good oral hygiene (OH) scores while 161 (67.9%) had a fair OH scores and 49 (20.7%) had poor OH scores (Figure 3). Distribution of oral hygiene scores of children from the different study centers: Five (83.3%) of the children at New Life had fair oral hygiene and one child (16.7%) had poor oral hygiene. However, only 2 (2.6%) of the children at Nyumbani had good oral hygiene, 65 (84.4%) fair oral hygiene and 10 (13%) poor oral hygiene. At Coast Province General Hospital, 21 (18.3%) had good oral hygiene, 70 (60.9%) fair oral hygiene and 24 (20.9%) had poor oral hygiene. Amongst children at Kenyatta National Hospital, 4 (10.3%) had good oral hygiene, 21 (53.8%) fair oral hygiene and 14 (35.9%) poor oral hygiene, however, statistical tests could not be conducted due to loss of statistical power. A comparison of the oral hygiene scores with the sociodemographic variables using the chi square test showed that statistically significant differences were noted between the OH scores among children from the homes and the outpatient centers. Only two children (2.4%) from the homes and 25 (16.2%) from the comprehensive care centers presented with good OH scores while 70 (84.3%) from the homes and 91 (59.1%) from the centers had fair OH scores. Eleven (13.3%) children from the homes had poor OH scores as opposed to 38 (24.7%) children from the centers and the difference was statistically significant with chi square 17.511; 2df; 0.000 at 95% CL. Table 1: Distribution of oral hygiene scores within the study centers n = 237.
Distribution of oral hygiene scores by gender
Eight (7.1%) boys and 19 (15.2%) girls exhibited good OH scores. Seventy-three (65.2%) males had fair OH, while 88 (70.4%) females were found to have fair oral hygiene. Among those with poor OH, 31 (27.7%) were boys while 18 (14.4%) were girls (Figure 4), the differences in the OH between the boys and the girls was significant chi square 641, 2df; p = 0.03 at 95% CL
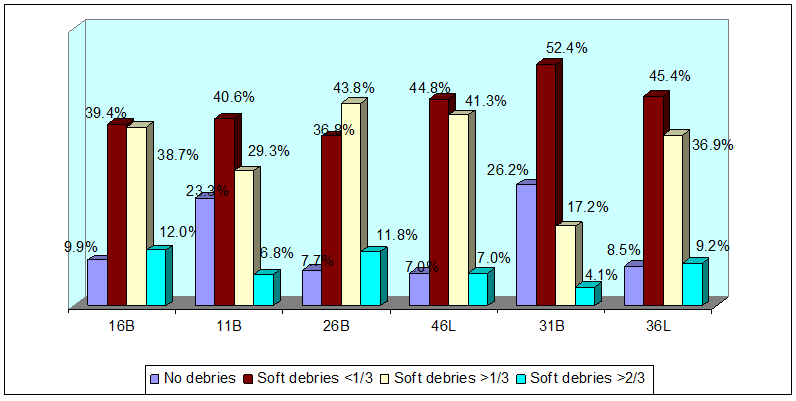
Distribution of plaque scores on the dentition
The deciduous molars 55, 65, 85, 75 had the highest distribution of plaque when compared to the incisors (Figure 5). A similar pattern of plaque distribution was also noted in the permanent dentition where the molars had a higher plaque accumulation compared to the incisors (Figure 6).
Oral hygiene scores and complaints of Pain
Among the children examined 98 (41.5%) complained of pain in the oral cavity while 139 (58.5%) did not. Out of those without pain, 16 (11.6%) had good OH scores, 104 (75.4%) fair OH and 18 (13%) poor OH. Those children with complaints of pain, only 11 (11.2%) had good OH and 56 (57.1%) fair OH while a relatively larger number (31,31.6%) had poor OH scores. Statistical tests for comparison were significant with a chi square 12.350; 2df; p = 0.002 at 95% CL.
Oral hygiene scores with challenges in maintaining oral hygiene
One hundred and forty-one (59.5%) children presented with no difficulties in maintaining oral hygiene while 96 (40.5%) did. Differences in oral hygiene scores between these two groups of children were noted to have been statistically significant 3.356; 2df; p = 0.001)}.
Oral hygiene with difficulties in feeding
Ninety-three (39.2%) children who complained of difficulties in feeding had lower scores of good (10, 10.4%) and fair oral hygiene (52, 33.9%); and higher scores of poor oral hygiene 31 (33.3%) whereas 144 (60.8%) who had no complaints had better oral hygiene scores. The chi square statistical test was significant at 15.172; 2df; p = 0.001 (Table 1).
Oral hygiene with ARV therapy: A total of 128 (54%) of the children were on antiretrovirals (ARVs). Among those on ARV 12 (9.4%) had good OH scores, 91 (71.1%) fair OH and 25 (19.5%) poor OH while among those not on ARV 15 (13.8%), 70 (64.2%) and 24 (22%) presented with good, fair and poor OH scores respectively. Statistical tests for comparison of oral hygiene with ARV treatment were not significant, chi 1.580; 2df; p = 0.454.
Oral hygiene with immunosuppression state
Among the children examined 90 (40.7%) had no evidence of immunosuppression, 67 (30.3%) had moderate suppression and 64 (29%) had severe suppression when categorized according to the CDC immune status classification. A test of statistical significance was noted between the hygiene scores and the immune status of the children with a greater number of children with no evidence of immunosuppression presenting with good and fair oral hygiene scores as opposed to those with moderate and severe suppression which was statistically significant with chi, square 13.028; 4df; p = 0.011.
Gingivitis with oral hygiene
Among the children with good oral hygiene scores, 18 (66.7%) did not have gingivitis and 9 (33.3%) presented with mild gingivitis while out of those with fair OH scores only 14 (8.7%) were free of gingivitis and 94 (58.4%) presented with mild and 53 (32.9%) moderate gingivitis respectively (Figure 7). Among the children with poor oral hygiene, only 12 (25%) had mild gingivitis though nearly three quarters of the children (36, 75%) presented with moderate gingivitis. The difference was highly significant Chi square 104.279, 4df = 0.000 at 95% CL.
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cursor on image to zoom/Click text to open image |
| |
Figure 6: Plaque scores in permanent dentition.
|
|
|
|
|
|
| Cursor on image to zoom/Click text to open image |

| |
Figure 7: Gingivitis and Oral Hygiene n = 236.
|
|
|
|
Gingivitis
|
|
Among the children examined 205 (86.5%) presented with gingivitis. One hundred and fifteen (48.5%) had mild gingivitis, 89 (37.6%) had moderate gingivitis and 1 (0.4%) child had severe gingivitis. Only 32 (13.5%) of the children were free of gingivitis.
Frequency of Brushing and Gingivitis: Among the children examined 202 (85.2%) reported brushing their teeth while 35 (14.8%) did not. Among the children who did not brush their teeth, 9 (26.5%) children did not present with gingivitis, while 10 (29.4%) presented with mild and 15 (44.1%) moderate gingivitis. Out of the children who reported brushing once daily, 18 (17.1%) were found to be free of gingival inflammation, 51 (48.1%) had mild gingivitis and 37 (34.9%) moderate gingivitis while among those children who reported brushing twice daily, 5 (5.2%) were free of gingivitis 54 (56.3%) had mild gingivitis and 37 (38.5%) moderate gingivitis. Statistical tests were found to be significant chi 14.368; 4df; p = 0.006.
Dental checkup and Gingivitis
Amongst the children who had never visited a dentist, 29 (21.3%) were free of gingivitis, 58 (42.6%) had mild gingivitis and 49 (36%) moderate gingivitis, while out of those who had visited a dentist only three children (3%) did not have gingival inflammation while 57 (57%) had mild and 40 (40%) moderate gingivitis respectively. The chi square was statistically significant 16.947; 2df; p = 0.000.
Distribution of gingivitis at the study centres: Among the children at New Life home 3 (50%) did not present with gingivitis, two (33.3%) had mild gingivitis and one (16.7%) child had moderate gingivitis. At Nyumbani children's' home, one (1.3%) child did not have gingivitis while 47 (61%) had mild and 29 (37.7%) moderate gingivitis respectively. At KNH 5 (12.8%) children were free of gingival inflammation, 13 (33.3%) presented with mild gingivitis, 20 (51.3%) moderate gingivitis and I (2.6%) severe gingivitis while at CPGH 23 (20%) had no gingivitis, 53 (46.1%) presented with mild gingivitis and 39 (33.9%) moderate gingivitis.
Among the children from the homes, 4 (4.8%) were free of gingivitis, 49 (59%) presented with mild gingivitis while 30 (36.1%) had moderate gingivitis as compared to 28 (18.3%) children at the C.C.C's who were found to have been free of gingivitis, 66 (43.1%) presenting with mild and 59 (38.6%) moderate gingivitis. The chi square test was found to be statistically significant 10.087; 2df; p = 0.006 at 95 % CL.
Distribution of gingivitis by gender
Eleven (9.8%) boys and 21 (16.9%) girls did not present with gingivitis while 51 (45.5%) boys and 64 (51.6%) girls presented with mild gingivitis and 50 (44.6%) boys and 39 (31.5%) girls had moderate gingivitis. Statistical tests however, showed no significant differences in the degree of gingivitis between the two genders chi square 5.385; 2df; p = 0.069 (Table 2).
Distribution of gingivitis by age: Among children in primary dentition (2–5 years) 19 (25.3%) were free of gingivitis, 35 (46.7%) had mild gingivitis and 22 (28%) moderate gingivitis respectively while out of a total number of 113 children aged 6–11 years, 8 (7.1%) were found not to have gingivitis, 53 (46.9%) had mild gingivitis and 52 (46%) moderate gingivitis. In the 12–15 years age group, only 5 (10.4%) adolescents had good gingival health while 27 (56.3%) presented with mild gingivitis and 16 (33.3%) moderate gingivitis respectively. The chi square was statistically significant; 16.403; 4df; p = 0.003 at 95% CL (Table 2).
Comparison of gender in the specific age subsets did not reveal statistically significant differences in the degree of gingivitis.
Ninety-eight children complained of pain in the mouth. Of these children only 14 (14.4%) were free of gingivitis while 38 (39.2%) had mild gingivitis and 46 (46.4%) had moderate gingivitis (Table 3). One hundred and thirty-eight children had no oral complaints of whom 18 (13%) had no gingivitis, 76 (55.1%) presented with mild gingivitis while 44 (31.9%) moderate gingivitis. Chi square test for comparison of gingivitis with oral complaints was significant chi square 6.214; 2df; p = 0.045 at 95% CL.
Gingivitis and challenges in maintaining oral hygiene
Among children who had no difficulties in maintaining oral hygiene 19 (13.5%) had no gingivitis, 76 (53.9%) presented with mild gingivitis and 46 (32.6%) moderate gingivitis while out of those who reported having difficulties only 13 (13.7%) were free of gingivitis, 39 (41.1%) had mild gingivitis and 43 (45.3%) moderate gingivitis (Table 3). Statistical tests were not significant. Chi square 4.329; 2df; p = 0.115 at 95% CL.
Gingivitis and difficulty in feeding
One hundred and forty-four (61%) children had no difficulty in feeding of whom 20 (13.9%) had no gingivitis, 79 (54.9%) presented with mild gingivitis and 45 (31.3%) moderate gingivitis (Table 3). Amongst 93 (39%) children who complained of feeding difficulty, 12 (13%) were free of gingivitis, 36 (52.2%) presented with mild and 45 (47.8%) moderate gingivitis respectively. There was a statistical significance difference chi square 6.970; 2df; p = 0.031.
Association between gingivitis and ARV therapy
Antiretroviral did not seem to influence gingivitis as out of 109 children who were not on therapy 17 (15.7%), did not present with gingivitis, 51 (47.2%) had mild and 41 (37.6%) moderate gingivitis respectively while among 127 children who were on therapy 15 (11.8%) were free of gingival inflammation, 63 (49.6%) presented with mild and 49 (38.6%) moderate gingivitis respectively. Chi square test was not significant 0.767; 2df; p = 0.681 at 95% CL.
Gingival status of children with Immune suppression
Absence of gingivitis was noted in 13 (14.4%) children with no evidence of immune suppression, 10 (14.9%) with moderate and 7 (11.1%) with severe suppression, however, mild gingivitis was present in 47 (52.2%) children with no evidence of suppression, 36 (53.7%) with moderate suppression and 23 (36.5%) with severe suppression. Moderate gingivitis was present in 30 (33.3%) children with no evidence of suppression, 21 (31.3%) with moderate suppression and 33 (52.4%) children with severe suppression. The chi square statistical test, however, was not significant 7.636; 4df; p = 0.106 at 95% CL.
|
|
|
|
Discussion
|
|
A large percentage of the children (67.9%) presented with fair oral hygiene though nearly a quarter of the study population had high plaque scores, concomitant with findings from studies by Gelbier et al. [9] where 51% (18/35) of the children examined had visible plaque deposits, Chen et al. [17]. who reported 64% moderate plaque accumulation and Riberio et al. [13]
where only 12.5% (7/56) of the children did not present with visible biofilm. Highly significant differences were noted regarding the oral hygiene among children from the homes and Comprehensive Care Centre (p = 0.000) with children from the homes exhibiting better oral hygiene. The plaque scores were higher compared to the general child population [11] [18] [19]
[20][21]. This could be due to lack of oral health knowledge, improper oral health practices, fear of brushing due to presence of oral lesions, financial restrictions coupled with the high cost of dental hygiene products and ignorance displayed by the parents/guardians towards oral health practices. Moreover the degree of immunosuppression, frequency of hospital admissions, illnesses due to HIV may also have contributed to the high plaque scores. In addition, the ARV medications could have been a contributory factor towards the poor oral hygiene due to their sticky nature and reported side effects of xerostomia [20] [21] [22] [23]. The girls had a better oral hygiene than boys (p = 0.013) as depicted in other studies on healthy children which could be explained by their oral hygiene practices [18] [24] [25]. The plaque scores did not vary among the three age groups examined in contrast to the study by Ramos et al. [10] reported that plaque scores increased as the age of the child increased. There was 59% of the children who presented with fair oral hygiene which was moderate plaque scores. A similar finding was also observed in the adolescents with 72.9% presenting with fair oral hygiene. This prevalence was still higher than that from local studies [19] [20] [21]. Highly significant differences in plaque scores were observed among children who reported brushing as opposed to those who did not (p = 0.000) thus indicating that oral hygiene practices are an important contributory factor towards good oral hygiene [15] [21]. Significant differences were also noted in the oral hygiene of children who had visited a dentist (p = 0.022). Oral hygiene may have been reinforced during dental visits and children may have been put on respective medications to alleviate complaints of oral pain hence allowing them to conduct thorough oral hygiene practices and maintain their oral hygiene [22]. In a longitudinal study of children infected with HIV reported high rates of dental disease and low adherence to referral for dental treatment. Okunseri et al. [23] also noted that although the HIV infected children could be considered as regular 'medical attendees', they were 'irregular dental attendees.' High plaque scores were noted among children who presented with positive complaints of oral pain (p = 0.002) and those who had challenges in maintaining oral hygiene and feeding (p = 0.001) hence reflecting on the possibility of pain and discomfort resulting in poor functioning of the oral cavity. Children with evidence of moderate and severe immunosuppression also presented with high plaque scores (p = 0.011). Similar findings were noted by Gelbier et al. [9] where out of 12 children who were moderately immunosuppressed, 6 presented with visible plaque deposits. This could be attributed to their poor state of health, frequent hospitalizations, frequent bouts of common illnesses and the presence of painful HIV related oral manifestations which arise when CD4 cell counts start diminishing as shown in various studies [24] [25][26][27][28]. However, in a study by Ramos et al. [29], there was no statistical significance between CD4 cell counts and plaque scores.
Gingivitis
The prevalence of gingivitis reported in this study was higher (86.5%) than studies conducted in Brazil, UK and USA which reported prevalence of 58.9% [13] and 60% [22] [30]respectively. Chen et al. [17] reported a prevalence of moderate gingivitis of 54% and a mean gingival index of 1.57. However, certain studies in the UK and US have quoted relatively lower conventional gingivitis prevalence of 45% [21], 40% [9] and 20.6% [12].Disparity in the prevalence may be attributed to the type of indices applied in the respective studies. The prevalence in the specific age groups examined were notably higher than those reported among various studies conducted on children from the general population in Kenya including handicapped children [11] [18] [19]. Among the adolescents 89.6% presented with gingivitis and this prevalence was notably higher than that quoted in a local study on 13–15 years old was 26% in Nairobi [19]. Literature suggests HIV infected children of all ages are at a greater risk for gingivitis and dental caries than children without HIV [22]. The prevalence of gingivitis varied significantly amongst the centres examined as did the plaque scores (p = 0.006). This again could be attributed to standard oral hygiene practices in the homes. There was no variation in the severity of gingivitis among the gender even though the girls had lower plaque scores than boys (p = 0.069). This finding could be attributed to hormonal changes in girls as they approach puberty which may exacerbate gingival inflammation despite them presenting with better oral hygiene. The degree of severity of gingivitis also varied significantly amongst the different age groups studied (p = 0.003), as children in the 6–11 years old cohort had the highest prevalence of moderate gingivitis. Similar findings were also noted in other studies. This could be related to the transition phase of shedding of deciduous teeth and eruption of permanent teeth. The relation between plaque and gingivitis was also highly significant (p = 0.000) concomitant with reports of Gelbier et al. [9], Riberio et al. [13] and Chen et al. [17]. Other than plaque, other factors seemed to influence the degree of gingivitis such as complaints of oral pain (p = 0.045) and difficulties in feeding (p = 0.031) which could have affected their oral hygiene practices and in turn resulted in an increased severity of gingival inflammation. Oral health practices were also found to have been statistically related to the degree of gingivitis (p = 0.006, p = 0.000) hence maintenance of good oral health practices cannot be over emphasized. Levels of immunosuppression was not statistically significant when associated with the severity of gingivitis (p = 0.106) though a pattern was observed. This finding was not in line with the overall consensus that the degree of immunosuppression affects the severity of gingivitis [9]
[10] [13][17], although further research may be required with larger samples to ascertain the finding.
|
Conclusion
|
|
This present study noted high prevalence of plaque scores, gingivitis and oral lesions among HIV/AIDS children. The levels of dental disease and HIV related oral lesion frequency was higher among children examined at the Comprehensive Care Centre. The plaque scores were notably higher in boys, children with poor oral health practices, oral complaints and increased immune suppression. The gingival scores were higher in the 6–11 year age group and severity of gingivitis was associated with higher plaque scores, deciduous dentition caries and increased number of oral manifestations. More oral lesions were also observed in children with higher plaque scores, increased severity of gingivitis and higher DMFT scores. Severity of dental disease and HIV related oral manifestations were also associated with poor oral hygiene practices, oral complaints and immune suppression states of the children.
|
Acknowledgements
|
|
We appreciate the contribution of Dr AbdulHalim Hussein for suggestions in the conception suggestions in the conception design and, guidance, encouragement of the research project. We are grateful to Miss Alice Lakati the biostatistician for data analysis. We appreciate the support given by the Chairman, Department of Pediatric Dentistry and Orthodontics and valuable peer contribution from faculty of the School of Dental Sciences during the research presentations. We acknowledge the ethical contributions and approval of the research by the Ethics and Research Standards Committee of the Kenyatta National Hospital and University of Nairobi for allowing me to conduct my research.
We are grateful to administration at the Nyumbani Children's Home, New Life.
Children's Home, the Comprehensive Care Centre, Kenyatta National Hospital and the Family Care Centre Coast Province General Hospital for allowing us to conduct the research at their facilities also for the help staff gave us during the research and the participation of the children.
|
References
|
-
Fleck F. WHO hopes 3-by-5 plan will reverse Africa's HIV/AIDS epidemic. Bull World Health Organ 2004 Jan;82(1):77–8.
[Pubmed]

-
UNAIDS. 2004 Report on the global AIDS epidemic, 4th global report. Geneva. 2004. [Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2004/GAR2004_en.pdf]

-
Central Bureau of Statistics Kenya, Ministry of Health Kenya, and ORC Macro. Kenya Demographic and Health Survey 2003. Calverton, Maryland: CBS, MOH, and ORC Macro. 2004. [Available at: http://www.dhsprogram.com/pubs/pdf/FR151/FR151.pdf]

-
World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. France: WHO; 2007. p. 2–52.
[Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf]

-
National AIDS and STI Control Programme. Ministry of Public Health & Sanitation. National guidelines for HIV testing and counselling in Kenya. Nairobi: NASCOP. 2008. [Available at: http://www.who.int/hiv/topics/vct/policy/KenyaGuidelines_Final2009.pdf]

-
Blake CM, Oxtoby MJ, Simonds RJ, Lindergren ML, Rogers MF. 1994 Revised classification system for human immunodeficiency Virus infection in children less than 13 years of age. Centre for disease control. MMWR. 1994;43(RR-12):1–10. [Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00032890.htm]

-
The World Oral Health Report 2003. Continuous improvement of oral health in the 21st century-the approach of the WHO Global Oral Health Programme, World Oral Health Organisation. 2003. [Available at: http://www.who.int/oral_health/media/en/orh_report03_en.pdf]

-
Scully C, McCarthy G. Management of oral health in persons with HIV infection. Oral Surg Oral Med Oral Pathol 1992 Feb;73(2):215–25.
[CrossRef]
[Pubmed]

-
Gelbier M, Lucas VS, Zervou NE, Roberts GJ, Novelli V. A preliminary investigation of dental disease in children with HIV infection. Int J Paediatr Dent 2000 Mar;10(1):13–8.
[CrossRef]
[Pubmed]

-
Ramos-Gomez FJ, Petru A, Hilton JF, Canchola AJ, Wara D, Greenspan JS. Oral manifestations and dental status in paediatric HIV infection. Int J Paediatr Dent 2000 Mar;10(1):3–11.
[CrossRef]
[Pubmed]

-
Masiga MA, Holt RD. The prevalence of dental caries and gingivitis and their relationship to social class amongst nursery-school children in Nairobi, Kenya. Int J Paediatr Dent 1993 Sep;3(3):135–40.
[CrossRef]
[Pubmed]

-
Okunseri C, Badner V, Wiznia A, Rosenberg M. Prevalence of oral lesions and percent CD4+, T-lymphocytes in HIV infected children on anti-retro viral therapy. AIDS Patient Care STDS 2003 Jan;17(1):5–11.
[CrossRef]
[Pubmed]

-
Ribeiro Ade A, Portela M, Souza IP. Relation between biofilm, caries activity and gingivitis in HIV+ children. [Article in Portuguese]. Pesqui Odontol Bras 2002 Apr–Jun;16(2):144–50.
[Pubmed]

-
Ministry of Health. Kenya national ART strategic plan 2003–2008. Kenya: Ministry of Health; 2003.

-
Havale R, Sheetal BS, Patil R, Hemant Kumar R, Anegundi RT, Inushekar KR. Dental notation for primary teeth: A review and suggestion of a novel system. Eur J Paediatr Dent 2015 Jun;16(2):163–6.
[Pubmed]

-
World Health Organisation. Oral health Surveys: Basic methods, 4ed. Geneva: World Health Organisation; 1997. p. 22–52.

-
Chen JW, Flaitz CM, Wullbrandt B, Sexton J. Association of dental health parameters with oral lesion prevalence in human immunodeficency virus-infected Romanian children. Pediatr Dent 2003 Sep–Oct;25(5):479–84.
[Pubmed]

-
Ng'ang'a PM, Valderhaug J. Oral hygiene practices and periodontal health in primary school children in Nairobi, Kenya. Acta Odontol Scand 1991 Oct;49(5):303–9.
[CrossRef]
[Pubmed]

-
Ng'ang'a PM. An overview of epidemiologic and related studies undertaken on common dental diseases and conditions in Kenya 1980-2000. Afr J Oral Hlth Sci 2002;1:103–10.

-
Reznick A. Perspective: Oral manifestations of HIV disease. J Int AIDS Soc 2006 Jan;13(5):143–8.

-
Hodge HC, Holloway PJ, Bell CR. Factors associated with toothbrushing behaviour in adolescents. Br Dent J 1982 Jan 19;152(2):49–51.
[Pubmed]

-
Broder HL, Russell SL, Varagiannis E, Reisine ST. Oral health perceptions and adherence with dental treatment referrals among caregivers of children with HIV. AIDS Educ Prev 1999 Dec;11(6):541–51.
[CrossRef]
[Pubmed]

-
Okunseri C, Badner V, Wiznia A, Rosenberg M. Dental visits by pediatric HIV-infected medical patients. N Y State Dent J
2003 Aug-Sep;69(7):26–9.
[Pubmed]

-
Howell RB, Jandinski JJ, Palumbo P, Shey Z, Houpt MI. Oral soft tissue manifestations and CD4 lymphocyte counts in HIV-infected children. Pediatr Dent 1996 Mar–Apr;18(2):117–20.
[Pubmed]

-
Ketchem L, Berkowitz RJ, McIlveen L, Forrester D, Rakusan T. Oral findings in HIV-seropositive children. Pediatr Dent 1990 May–Jun;12(3):143–6.
[Pubmed]

-
Katz MH, Mastrucci MT, Leggott PJ, Westenhouse J, Greenspan JS, Scott GB. Prognostic significance of oral lesions in children with perinatally acquired human immunodeficiency virus infection. Am J Dis Child 1993 Jan;147(1):45–8.
[CrossRef]
[Pubmed]

-
Moniaci D, Cavallari M, Greco D, et al. Oral lesions in children born to HIV-1 positive women. J Oral Pathol Med 1993 Jan;22(1):8–11.
[CrossRef]
[Pubmed]

-
Del Toro A, Berkowitz R, Meyerowitz C, Frenkel LM. Oral findings in asymptomatic (P-1) and symptomatic (P-2) HIV infected children. Pediatr Dent 1996 Mar–Apr;18(2):114–6.
[Pubmed]

-
Ramos-Gomez FJ, Hilton JF, Canchola AJ, Greenspan D, Greenspan JS, Maldonado YA. Risk factors for HIV-related orofacial soft-tissue manifestations in children. Pediatr Dent 1996 Mar–Apr;18(2):121–6.
[Pubmed]

-
Santos LC, Castro GF, de Souza IP, Oliveira RH. Oral manifestations related to immunosuppression degree in HIV-positive children. Braz Dent J 2001;12(2):135–8.
[Pubmed]

|

