|
Case Report
A novel treatment of necrotizing ulcerative gingivitis using ozone therapy
1 Dentalogy Tooth care centre A18, Hermes Kunj Mangaldas Road opposite Conrad Hotel Near Taj Vivanta, Pune, Maharashtra, India
2 Senior Consultant, D.Y. Patil Vidyapeeth, Dental College & Hospital, Department of Periodontology, India
3 Private practice, India
4 MDS, Assistant Professor, Department of Oral Pathology and Microbiology, D.J. College of Dental Sciences and Research, Modinagar, Ghaziabad, Uttar Pradesh, India
Address correspondence to:
Ketkee P Asnani
Dentalogy Tooth care centre A18, Hermes Kunj Mangaldas Road opposite Conrad Hotel Near Taj Vivanta, Pune, Maharashtra,
India
Message to Corresponding Author
Article ID: 100037D01KA2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Asnani KP, Kaur G, Hingorani D, Sekhon HK. A novel treatment of necrotizing ulcerative gingivitis using ozone therapy. Edorium J Dent 2019;6:100037D01KA2019.ABSTRACT
Introduction: Necrotising ulcerative gingivitis (NUG) is a microbial disease characterized by the death and sloughing of gingival tissue. This article presents a classic case with characteristic symptoms of gingival necrosis, punched out ulcerated papillae, pseudomembrane formation and pain.
Case Report: The patient was treated with the conventional treatment modalities of antimicrobial treatment, swabbing of the pseudomembrane with H2O2 followed by irrigation at home mouth rinses. A soft toothbrush was advised without any adjunctive toothpaste. Additionally, ozone therapy was done on the 3rd day since the lesion persisted. Each arch was ozonated for five minutes using custom made silicon stents with nozzle in the front to allow the ozone generator to pass through. With adjunctive ozone therapy lesions had started to resolve by the 5th day and the patient was also relieved of all associated symptoms.
Conclusion: Elimination of the bacteria is a very important part of treatment of NUG. Treponema, bacteroides intermedius and fusobacterium species are the principle bacteria involved in the disease. These bacteria are essentially anaerobic. Ozone therapy creates an aerobic environment for these bacteria inhibiting their growth and disrupting the integrity of the bacterial cell wall by oxidation and inactivating the existing bacteria. Ozone therapy does not damage the other surrounding healthy cells. These healthy cells have a coating of enzymes like catalase, glutathione perioxidase, reductase and superoxide dimutase which prevents the oxygen radical from penetrating them. Hence using ozone as an adjunctive therapy helps accelerate healing and treat the basic etiology in a very targeted manner.
Keywords: Bacteria, Necrotising ulcerative gingivitis, Ozone therapy, Periodental disease
INTRODUCTION
Necrotizing ulcerative gingivitis (NUG) is a distinct and specific disease which is rapidly destructive, noncommunicable and of complex etiology. It is classified as “Necrotizing Periodontal Disease” according to the 1999 American Academy of Periodontology classification system [1].
Necrotizing ulcerative gingivitis is different from the other periodontal diseases, in that it presents with interdental gingival necrosis leading to “punched out” ulcerated papillae, spontaneous gingival bleeding and pain with pseudomembrane formation [2],[3]. Patients usually present with constant, severe, radiating and gnawing pain generally following an episode of systemic disease, increased physical demand, reduced nutrition intake or psychological stress. Fetid breath and pseudomembrane are secondary diagnostic features [4].
Etiology of NUG is complex, with no direct cause-effect relation that has yet been established [5]. Accumulation of bacteria due to poor oral hygiene, overhanging margins and restorations, food impaction, malpositioned teeth and calculus; cigarette smoking; decreased host resistance act as prerequisites for development of NUG [6]. Treponema pallidum-related spirochetes have been reported to be associated with the condition [7]. The molecular and cellular mechanism involved in the pathogenesis have still not been elucidated, however, the neutrophils are thought to be mediating cytokine induced tissue destruction [3],[8].
Conventional treatment of NUG involves oral hygiene reinforcement, mechanical debridement of plaque, vitamin and diet therapy, oxygenating agents, holistic care, and administration of antibiotics and their various combinations [5]. The antibiotics and oxygenating agents are thought to eliminate the bacterial etiology, while vitamin therapy acts as an adjunct to overcome nutritional deficiencies. However, these conventional therapies fail to completely eradicate the causative microorganisms.
An oxygen rich environment can always supplement antibiotic therapy by negatively affecting the anaerobic microorganisms [9]. Further advantage of oxygen therapy is that it can be delivered locally which helps in easy diffusion into the mucosa and cells. Local oxygen delivery to an infectious area also leads to accelerated healing process in any part of the body [10]. This applies specifically to areas with poorly healing superficial wounds and impaired mucosal integrity as seen in NUG.
Ozone is a triatomic molecule consisting of three oxygen atoms and is widely used in medicine and dentistry due to its antimicrobial, antihypoxic, analgesic and immunostimulating effects on the biological systems [11]. This case report demonstrates the apparent beneficial use of topical ozone application in a patient with NUG.
CASE REPORT
An unmarried 21-year-old female presented with a chief complaint of acute, constant, radiating pain in her jaws. The pain was so severe that it impaired her brushing and eating activities. She also presented general symptoms of fever and malaise. Detailed personal history revealed that the patient was undergoing emotional stress and was suffering from sleep deprivation due to her upcoming examinations. Her oral hygiene was fair with slight plaque accumulation and halitosis. She was a nonsmoker and otherwise systemically healthy.
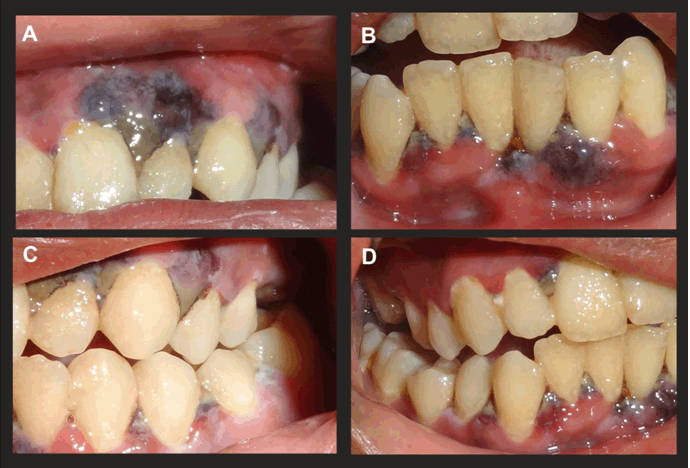
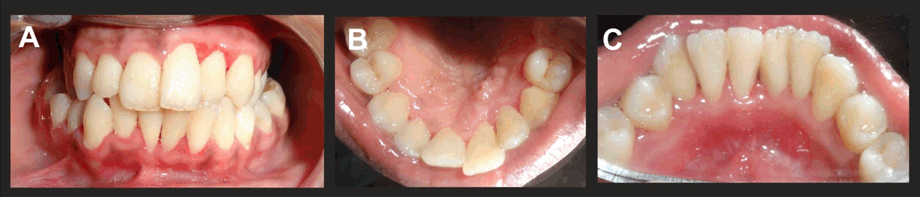
On intraoral examination, a typical pseudomembranous slough was seen extending from tooth numbers 4–13 and tooth numbers 20-29. Marked gingival necrosis was seen with marginal and interdental gingiva, which also spread to the attached gingival region (Figure 1A–D).
Antimicrobial treatment was constituted immediately which comprised of antibiotic Amoxicillin 500 mg thrice a day for seven days, Metronidazole 400 mg twice a day for seven days. Patient was also prescribed an analgesic, combiflam (ibuprofen 400 mg and paracetamol 325 mg) thrice a day for seven days. In office treatment comprised of swabbing the pseudomembrane with 3% hydrogen peroxide (H2O2) followed by irrigation with the same. Patient was also prescribed rinses with H2O2 to be done at home 4–5 times per day. Mechanical plaque control using a soft toothbrush of 0.006” diameter bristles was advised without any adjunctive toothpaste. Patient was kept on a daily recall.
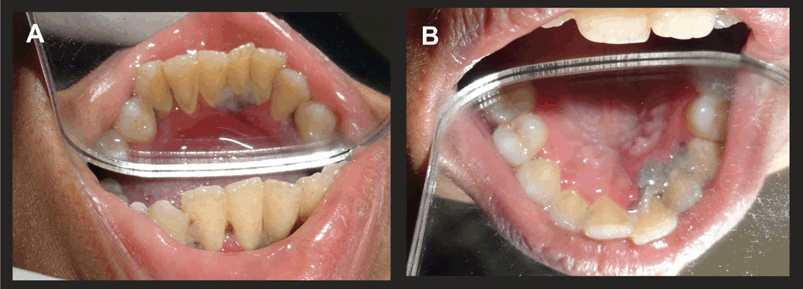
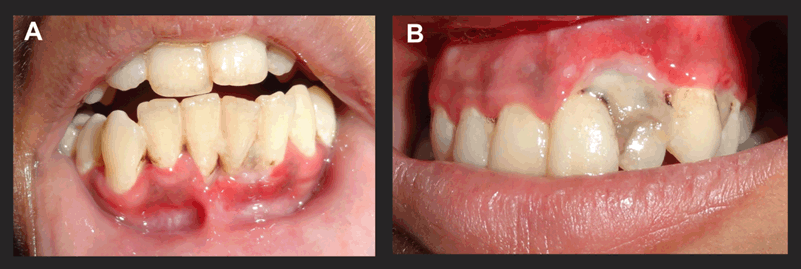
On the second day the lesions had acquired a whitish hue. However, lesions had also started appearing palatally with respect to tooth numbers 9, 10, 11 (Figure 2A) and lingually w.r.t 22, 23, 24 (Figure 2B). The mandibular gingiva acquired a fiery red colour and peeling of the pseudomembrane was visible on swabbing, leaving behind a raw bleeding surface. Antibiotics and H2O2 were continued.
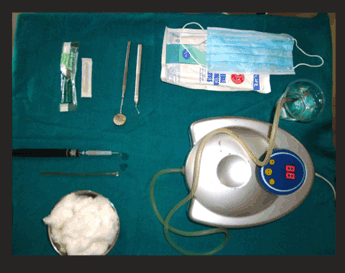
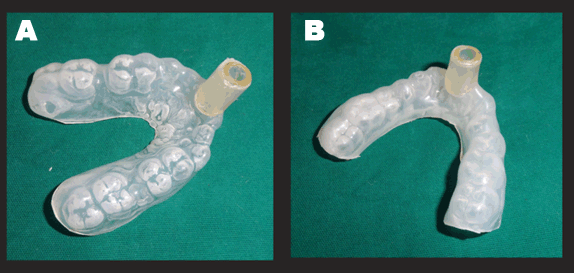
On day three, the whitish hue persisted (Figure 3A and B) and it was decided to constitute ozone therapy (Figure 4) as a part of the treatment. Custom made silicon stents were used for its delivery (Figure 5A, B). The stents had a small nozzle in the front to allow the ozone generator tube to pass through and channel the ozone to both the arches. Each arch was ozonated for five minutes.
Ozone treatment was continued on day 4 and 5. By the 5th day the lesions had started to resolve and the patient also was relieved of all the associated symptoms. Scaling and root planing was done on fifth day (Figure 6A–C).
DISCUSSION
First reference of oral disease distinguished by the typical clinical signs and symptoms of NUG was reported in the historical war records of Xenophon’s troops during the fourth century BC. John Hunter, in 1778, was the first person to delineate the clinical differences between NUG, scurvy and chronic periodontitis [12]. Hirsch added secondary features to the clinical presentation of NUG in 1886, while Plaut in 1894 and Vincent in 1896 were the first to attribute the origin of the lesion to fusiform and spirochetal bacteria [13], [14].
Necrotizing ulcerative gingivitis can cause tissue destruction involving the supporting structures. It usually runs an acute course and therefore the term acute is often included in the diagnosis. When bone loss occurs the condition is called necrotizing ulcerative periodontitis.
The three essential aims behind initial treatment of NUG are elimination of disease to prevent continuing tissue loss laterally and apically, relieving the patient of pain and unease in order to enable the patient to resume the function of mastication and alleviating the general symptoms of illness [15]. Keeping in mind the bacterial etiology of NUG, antibiotics seem to be the most logical treatment. Antibiotics are a strict recommendation for patients with systemic signs and symptoms. Penicillin, owing to its broad spectrum of action, is the drug of choice. However, a relapse of the condition as a chronic one is frequently encountered with its use [16]. Hence combination protocol incorporating amoxicillin and metronidazole is frequently used. Metronidazole prescribed is usually effective in alleviating the acute phase of NUG [17]. It decreases the proportions of Treponema, Bacteroides intermedius and Fusobacterium species for two-three months post treatment [18].
Along with systemic, local treatment is required to eliminate the contributing factors and gingival deformities. Local therapy constituted of H2O2 swabbings, scaling and root planing on fifth day, and use of a soft dry toothbrush. Hence, systemic antibiotics are considered only as adjunct treatment and not a substitute for local therapy.
Ozone, which is used for medical purposes, is a gas mixture comprised of 95 to 99.95% oxygen and 0.05 to 5% pure ozone. In dentistry, ozone therapy was first evaluated in 1933 for treatment of mucosal lesion. Ozone therapy works on the basic principle of creating an aerobic environment for the anerobic pathogenic bacteria, thus inhibiting their growth. It disrupts the integrity of the bacterial cell wall by oxidation and inactivates the existing bacteria [19]. Its oxidizing capacity due to formation of free radical is lethal towards almost all anaerobic microorganisms [20], [21]. It damages the cytoplasmic membrane due to ozonolysis of dual bonds and oxidation of protein causing loss of organelle function. Its function is selective to microbial cells and does not damage human body cell since they possess antioxidative ability. Healthy cells have a coating of enzymes like catalase, glutathione peroxidise, reductase and superoxide dimutase which prevent oxygen radical from penetrating them. Unhealthy cells do not have these enzyme coatings; therefore they are destroyed by ozone. Thus, ozone therapy provides a very target specific approach to the condition [19]. Furthermore, increased oxygen tension accelerates regenerative therapy by increasing tissue capillarity and fibroblast replication [22].
At humoral and cellular level, it stimulates proliferation of immunocompetent cells and synthesis of immunoglobulins. It increases phagocytic activity by increasing function of fibroblasts. There is an increase in the ribosomal activity causing increased protein synthesis of prostaglandins, interleukins and leukotrines that help in resolution of inflammation [11].
Due to the aforementioned benefits of ozone therapy, it was decided to use the same for treatment of our case of NUG. The local debridement and proper oral hygiene practice with antibiotic coverage healed the lesion considerably. The healing was seen accelerated post the application of ozone on the third day. Patient was relieved from pain, swollen gingiva and fetid odor completely with this approach.
Keeping in mind this case presentation, studies with larger sample size and further microbiological analysis are warranted for correct assessment. Lack of evaluation of microbial profile is a limitation of this case report.
CONCLUSION
Adjunctive ozone therapy accelerates the reduction in microorganisms and the illness can be brought under control more quickly. It is very target specific and acts effectively against the main pathogenic bacteria causing this condition. Hence, it can be a very promising treatment option for lesions like necrotising ulcerative gingivitis.
REFERENCES
1.
Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4(1):1–6. [CrossRef]
[Pubmed]

2.
Lang N, Soskolne WA, Greenstein G, et al. Consensus report: Necrotizing periodontal diseases. Ann Periodontol 1999;4(1):78. [CrossRef]

3.
4.
5.
Murayama Y, Kurihara H, Nagai A, Dompkowski D, Van Dyke TE. Acute necrotizing ulcerative gingivitis: Risk factors involving host defense mechanisms. Periodontol 2000 1994;6:116–24.
[Pubmed]

6.
May OA Jr. Acute necrotizing ulcerative gingivitis: A review. Va Dent J 1984;61(5):7–12.
[Pubmed]

7.
Riviere GR, Weisz KS, Simonson LG, Lukehart SA. Pathogen-related spirochetes identified within gingival tissue from patients with acute necrotizing ulcerative gingivitis. Infect Immun 1991;59(8):2653–57.
[Pubmed]

8.
Cogen RB, Stevens AW Jr, Cohen-Cole S, Kirk K, Freeman A. Leukocyte function in the etiology of acute necrotizing ulcerative gingivitis. J Periodontol 1983;54(7):402–7. [CrossRef]
[Pubmed]

9.
Larsson A, Engström M, Uusijärvi J, Kihlström L, Lind F, Mathiesen T. Hyperbaric oxygen treatment of postoperative neurosurgical infections. Neurosurgery 2002;50(2):287–95.
[Pubmed]

10.
Wang C, Schwaitzberg S, Berliner E, Zarin DA, Lau J. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch Surg 2003;138(3):272–9.
[Pubmed]

11.
Seidler V, Linetskiy I, Hubálková H, Stanková H, Smucler R, Mazánek J. Ozone and its usage in general medicine and dentistry. A review article. Prague Med Rep 2008;109(1):5–13.
[Pubmed]

12.
13.
14.
15.
16.
Schluger S. Necrotizing ulcerative gingivitis in the Army; incidence, communicability and treatment. J Am Dent Assoc 1949;38(2):174–83. [CrossRef]
[Pubmed]

17.
Wade AB, Blake GC, Mirza KB. Effectiveness of metronidazole in treating the acute phase of ulcerative gingivitis. Dent Pract Dent Rec 1966;16(12):440–3.
[Pubmed]

18.
Loesche WJ, Syed SA, Laughon BE, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol 1982;53(4):223–30. [CrossRef]
[Pubmed]

20.
Broadwater WT, Hoehn RC, King PH. Sensitivity of three selected bacterial species to ozone. Appl Microbiol 1973;26(3):391–3.
[Pubmed]

21.
22.
Tompach PC, Lew D, Stoll JL. Cell response to hyperbaric oxygen treatment. Int J Oral Maxillofac Surg 1997;26(2):82–6.
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ketkee P Asnani - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gurbani Kaur - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dinesh Hingorani - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Harjeet Kaur Sekhon - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Ketkee P Asnani et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.