| Table of Contents |  |
|
Original Article
| ||||||
| Antibacterial properties of new calcium based cement prepared from egg shell | ||||||
| Emad F. Alkhalidi1, Talal H. Alsalman1, Amer A. Taqa2 | ||||||
|
1Department of Conservative Dentistry, College of Dentistry, University of Mosul.
2Department of DBS, College of Dentistry, University of Mosul. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Alkhalidi EF, Alsalman TH, Taqa AA. Antibacterial properties of new calcium based cement prepared from egg shell. Edorium J Dent 2015;2:21–28. |
|
Abstract
|
|
Aims:
The study was conducted to evaluate the pH and antimicrobial properties of newly prepared calcium based cement, polycarboxylate cement and biodentine material.
Methods: The new cement was prepared from egg shell, for pH measurements, 9 standard discs (6 mm in diameter and 2 mm in thickness) were prepared for each material, The antibacterial effects of set specimens against Streptococcus mutans, oral Lactobacillus and Enterococcus faecalis were assessed by agar diffusion tests, 90 discs shaped specimens (30 of each type of materials; 6 mm in diameter x 2 mm in thickness) were prepared. Three discs, one for each material was placed on each agar plate, and the plates incubated for 24 hours. After incubation, the diameter of inhibition zone was measured at three different points and the mean value was used as the result of the specimen. Results: Statistically significant differences were identified between cements, calcium based cement and biodentine exhibited higher mean values of pH than polycarboxylate cement, ANOVA and Duncan's multiple range test were done to evaluate the effect of materials against each type of bacteria, it showed that the inhibition zones produced by experimental cement were statistically significantly larger than those produced by the other materials. Conclusion: Within the limitation of this research, it was concluded that new calcium based cement has a better antimicrobial properties than biodentine and polycarboxylate cement. | |
|
Keywords:
Antimicrobial, Biodentine, Calcium based cement, Polycarboxylate cement
| |
|
Introduction
| ||||||
|
Secondary caries is the main reason for the failure and, therefore, replacement of dental restorations; this may be caused by insufficient oral hygiene, bacterial microleakage, residual bacteria remaining in the cavity preparation after treatment, or a combination of these causes [1] [2]. Carious lesions are formed of two layers; an outer heavily infected layer that must be removed during cavity preparation and an inner affected layer that would be retained and treated with a therapeutic lining or base [3]. Due to high frequency of recurrent caries after restorative treatment, great attention has been paid to the therapeutic effects revealed by direct filling materials. The antibacterial effect is an essential property because inactivation of bacteria means a direct strategy to eliminate the cause of dental caries [4] [5]. Dental cements play a key role in assuring a healthy, infection free oral cavity as they allow the sealing of damaged areas. However, it is still likely for infections to occur within these cements and as such, one of the key properties of dental cements must be antimicrobial activity [6] [7]. Dental cements are commonly used in odontological treatment, However, due to the hazardous nature and reduced biological efficiency of some of the used materials, newer and safer substitutions are needed, particularly so those possessing higher antimicrobial activity than their traditional complements [8] [9] [10]. It has been shown that cements containing calcium hydroxide could provide antibacterial activity and sterilize carious dentin [11] [12]. Among the major contaminants of dental cements we have several species in the oral cavity such as Enterococcus, Lactobacillus, and Streptococcus species. The inability to inhibit these species may lead to tissue invasion with consequential pulpal necrosis and tooth loss, thus, the search for new compounds that may improve the biocompatibility and functionality with the antibacterial properties of the cements has gained importance [13] . Antimicrobial action and ability to induce formation of mineralized tissue are both dependent on alkaline pH [14]. The aim of this study was to evaluate and compare the antimicrobial action and pH changes promoted by new calcium based cement (prepared from egg shells) and compare the result with polycarboxylate cement and biodentine material. | ||||||
|
Materials and Methods
| ||||||
|
In this study, we had three experimental groups as follows:
pH Analysis Measurement of pH was performed with a pH meter (Weilheim, Germany), previously calibrated with buffer solutions of known pH (4, 7 and 10), at constant room temperature (25°C). After calibration of the pH meter the pH was determined good stirring of the solution for 5 seconds, then insertion of pH electrode in every sample studied, and measuring on the pH value on the digital screen [16] [17] [18] (calibrate the pH meter after every reading for standardization). Antimicrobial study The antibacterial effects of set specimens against Streptococcus mutans, oral Lactobacilli and Enterococcus faecalis were assessed by agar diffusion tests as described by Bauer et al. [19]. A single colony from each type of bacteria after isolation and identification transferred into (5 mL) sterile BHI broth (Lab, UK) and incubated at 37°C for 24 hours. In order to prepare the experimental suspensions, McFarland 0.5 turbidity tube was prepared, and used to prepare suspensions of the strains in brain heart infusion at approximately 1.5×108 organisms/mL, which were flooded-inoculated onto the surface of Muller-Hinton agar (Lab, UK) plates [19]. Thirty Petri plates with 20 mL of Muller-Hinton agar were inoculated with the microbial suspensions using sterile swabs that were spread on the medium. 90 discs shaped specimens (30 of each type of materials; 6 mm in diameter x 2 mm in thickness) were prepared and divided into three major groups:
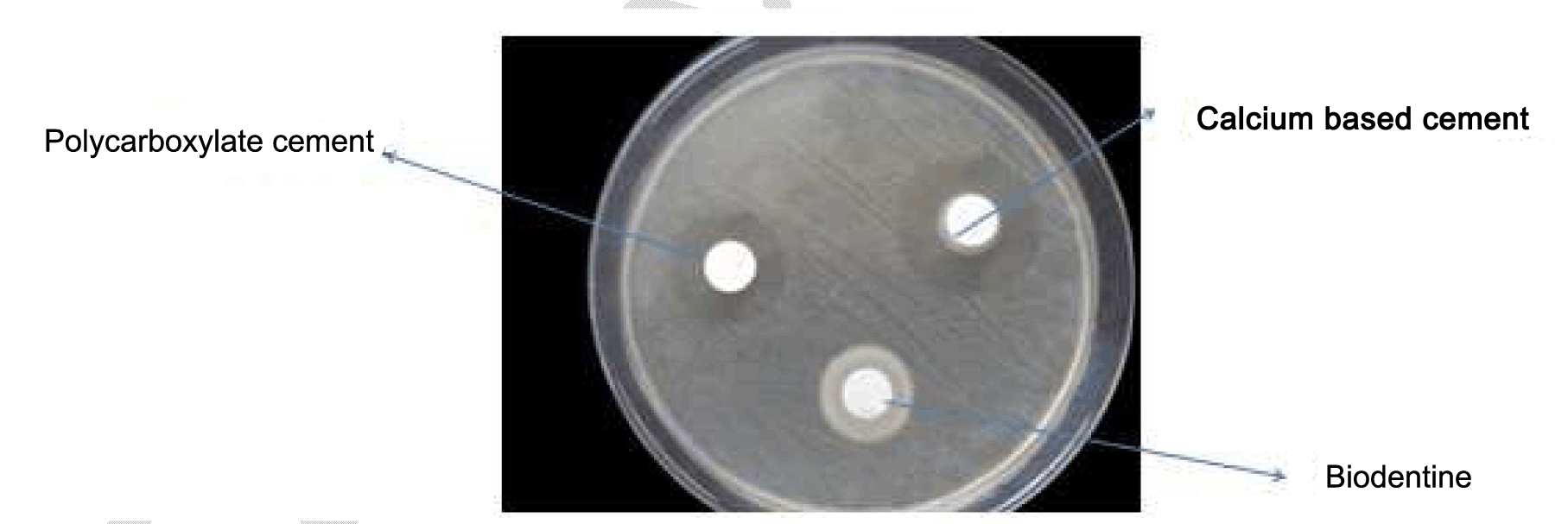
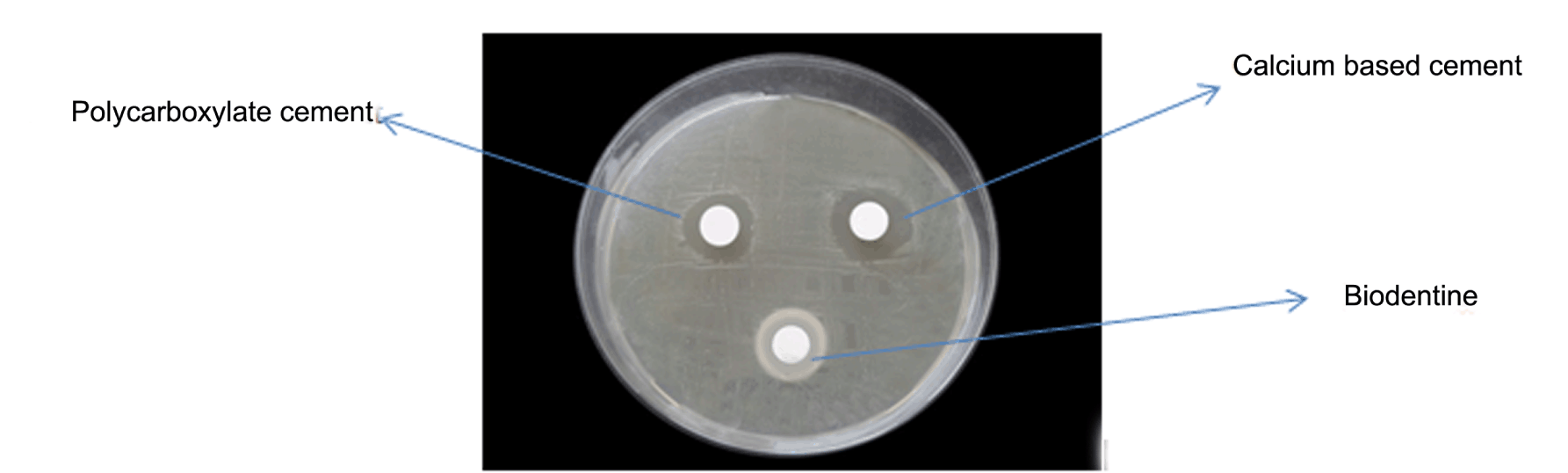
Three discs, one for each material was placed by a sterile forceps on each agar plate, and incubated in an aerobic candle jar at 37C° for 24 hours. After incubation, the diameter of inhibition zone was measured at three different points and the mean value was used as the result of the specimen, the size of the inhibition zones for each material was calculated from the diameters of the halo of inhibition produced and the disc's diameter as follows: Size of inhibition zone = diameter of halo-diameter of the disc (Figure 1) (Figure 2) (Figure 3). | ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Results | ||||||
|
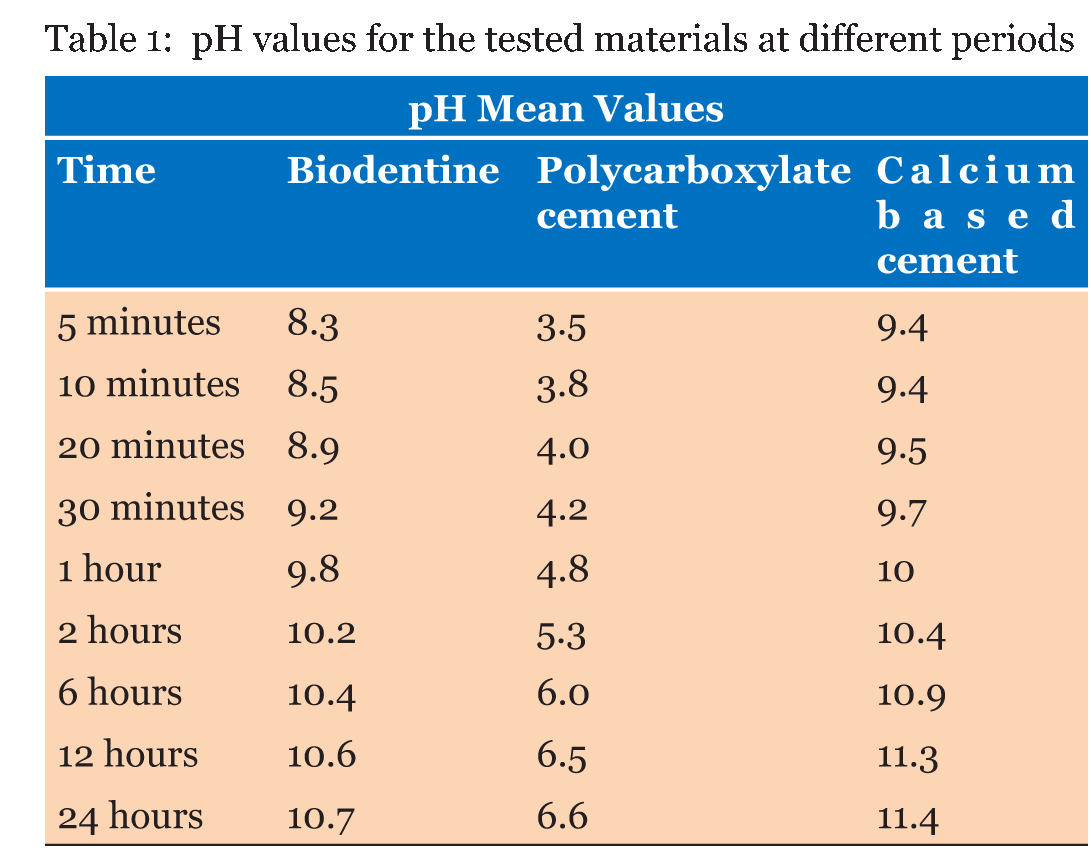
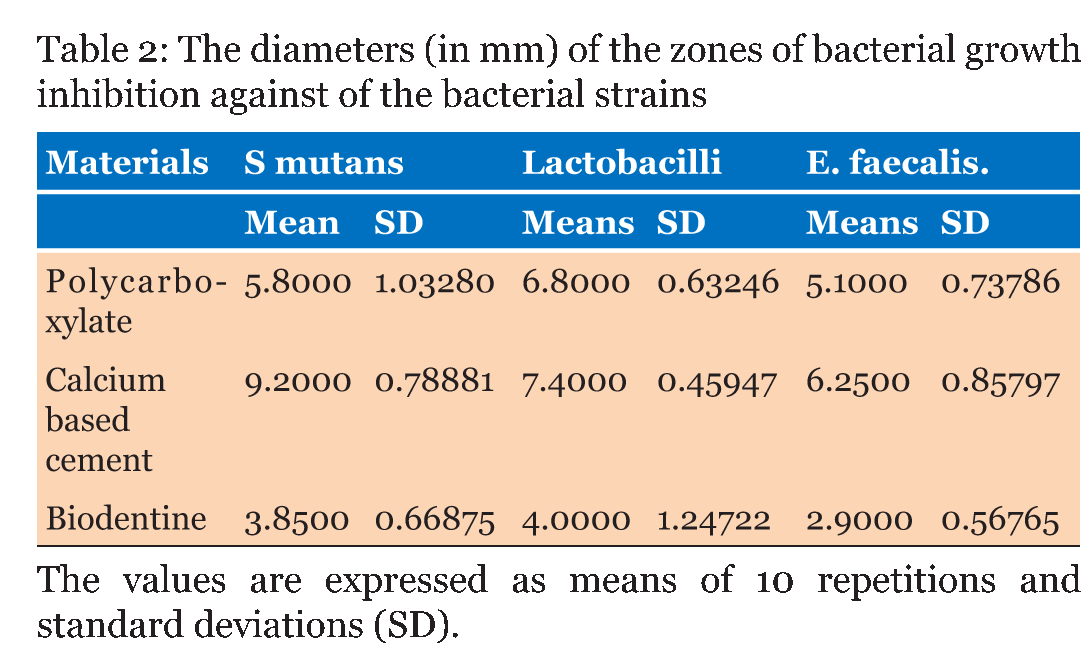
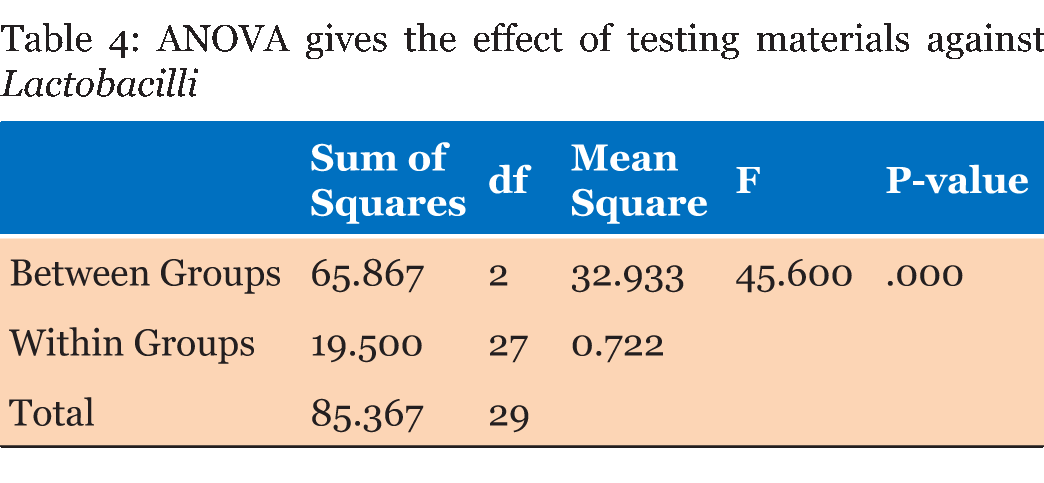
Table 1 gives the means of the pH mean values of the materials at each experimental period. From the result, it can demonstrate that the calcium based cement and biodentine give alkaline reaction and show increase in pH within time. Calcium based cement give pH 9.4 after five minutes from reaction and end in pH 11.4 after 24 hours of reaction, biodentine give pH start with 8.3 and over 24 hours the pH reach 10.7. While zinc polycarboxylate cement presented the lowest pH values (acidic reaction) at all experimental periods, it starts with pH 3.5 and after 24 hours it reaches pH 6.6. Table 2 gives the diameters (means and standard deviations) of the inhibition zones of bacterial growth (in mm) obtained for the tested materials. The ANOVA test shows that there were statistically significant differences (p < 0.0001) among the inhibition zones produced by the tested materials for all bacterial strains (Table 3) (Table 4) (Table 5) . | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
The durability of dental restorations is frequently determined by their ability to resist plaque formation and subsequently avoid secondary caries. Cements with antibacterial properties could successfully prevent or delay caries formation and extend the lifetime of restorations. In this study, the antibacterial properties of different classes of dental materials were investigated by agar diffusion test [20] [21] [22]. The agar diffusion test is a routinely used method to investigate the antimicrobial properties of dental materials. This method involves placing the tested material on an agar plate inoculated with oral bacteria. By this test method, an inhibition zone around, the material is produced. To produce the zone of inhibition, the material needs to be able to leach a soluble antimicrobial agent. If the elution is not of adequate amounts of antimicrobial agent the zone of inhibition will not be produced. In general, larger zones correlate with concentration and/or potency of testing bactericide as well as susceptible to tested bacteria to specific antimicrobial agent, the size of inhibition is measured using graduated ruler [23] [24] [25]. The bacterial strains used throughout the experiments were Streptococcus mutans, oral Lactobacilli and Enterococcus fecalis these microorganisms play a major role in dental biofilm formation and in the etiology and progression of caries. Streptococcus mutans is one of the bacteria most frequently implicated in dental caries, it is efficiently degrading fermentable carbohydrates to acids, which can demineralize tooth tissue [26] [27] [28] [29]. Lactobacilli have previously been found to be present at the advancing front of a progressive carious lesion as well as prominent members of the microbial community in the initial stages of root canal infection. Lactobacillus spp have been demonstrated to bind to type 1 collagen (Liu et al. 1991, Love and Jenkinson 2002) suggesting that they would be able to attach to exposed dentine within the carious lesion and form biofilms. During biofilm development, bacteria undergo physiological adaptation, which can result in as much as 50% of the bacterial protein being differentialy produced as compared to free-floating cells [30] [31] . Fransson et al. 2014 concluded that the E. faecalis reduced activity of odontoblast-like cells, E. faecalis had an inhibitory effect on collagen 1 production, so it decreases the ability of odontoblasts to initiate synthesis of tertiary dentine [32]. E. faecalis also has been shown to be a highly resistant bacteria in the root canal system, and it plays an important role in endodontic treatment failures [33]. Our results showed effective antibacterial activity of experimental calcium based cement which caused greater growth inhibited zones of testing bacteria than polycarboxylate cement and biodentine, which may be related to the liberation of ions and alkalinity of calcium based cement. Research showed that bacteria tolerate well in the pH range from 4.5–8.5. The pH of experimental calcium based cement can reach over 11 during the reaction, the alkalinity of calcium based cement are essential for the antimicrobial effect [34]. Estrela et al. (1995) showed that pH greater than nine may reversibly or irreversibly inactive cellular membrane enzymes of the microorganism resulting in a loss of biological activity [35]. The experimental cement consists of (70% CaO, and 25% MgO, 3% hydroxyappetite, 1.5% bismuth oxide, 0.5% calcium acetate). Kouassi et al. (2003) show that CaO-based cement has an antibacterial effect and is a potential candidate for pulp capping and cavity lining [36]. Li et al. (1998) found that hydroxyapatite (HA) has antibacterial properties against cariogenic bacteria and they conclude that it would be best to harness the antibacterial property of HA, by using it as a base in the treatment of carious cavities, to act against residual cariogenic bacteria [37]. Tin-oo et al. 2007 show that Tubes containing 200 mg of HA or more showed complete inhibition of S. mutans, as no bacterial growth was found. This finding has shown that HA owns antibacterial properties. This study has demonstrated that HA has antibacterial activity, against cariogenic bacteria (S. mutans) so the HA can be used as a prospective candidate for dental applications such as pulp capping or as a base liner material [38] . The bacterial growth inhibition could be attributed also to the magnesium ions or the active oxygen released by MgO into the medium [39]. (Sawai et al. 1995a) they showed that the Magnesium oxide (MgO), calcium oxide (CaO) and zinc oxide (ZnO) exhibited strong antibacterial activity. They found that the magnesium oxide and calcium oxide powders acted against both gram-positive and gram-negative bacteria in a bactericidal manner, while zinc oxide powder inhibited the growth of gram positive bacteria more strongly than a gram negative bacteria [40]. Conventional cements that set of acid-base interactions produce an environment that is initially acidic, but approach neutrality during the course of the reaction. Low primary pH is recognized as beneficial with regards to bactericidal properties [41]. Our study showed that polycarboxylate cement be active antibacterial effective against all experimental types of bacteria, which was attributed to low pH. Polycarboxylate cement consists of mainly Zn which is about 90% and 10% MgO both of which have antimicrobial activity, Zinc acts as an inhibitor of multiple activities in the bacterial cell, such as glycolysis, trans membrane proton translocation and acid tolerance. Consequently, zinc is normally described as acting in high concentrations, bacteriostatic rather than bactericidal agent [42] [43][44]. Biodentine showed some initial bacterial inhibition but was significantly lower than experimental calcium based cement and polycarboxylate which displayed a large spread in data. The fact that Biodentine showed zones of inhibition implies that Biodentine itself has some limited antimicrobial effects and might be attributed to Biodentine high pH [45]. However, the smaller inhibition zone of biodentine against E. faecalis is related to the fact that Enterococcus faecalis can survive in alkaline environments (up to pH of 11.1). Perhaps the inherent, persistent alkalinity of Biodentine is just enough to overwhelm the E. faecalis [32]. Biodentine powder is composed mainly from tricalcium silicate, calcium carbonate, and zirconium oxide as the radio-pacifier, whilst Biodentine liquid contains calcium chloride as the setting accelerator and water as reducing agent. The addition of up to 30% calcium carbonate, calcium sulfate, and calcium chloride resulted in improvement in physical properties of tricalcium silicate cement as well as improving the degradability and bioactivity of the resultant material. Hydration of tricalcium silicate results in the formation of calcium silicate hydrate gel, calcium hydroxide, and unreacted tricalcium silicate. The calcium hydroxide produced from the tricalcium silicate hydration has antibacterial and anti-inflammatory properties, mainly due to the high (alkaline) pH of the surrounding environment after it dissolves [45] [46][47]. | ||||||
|
Conclusion
| ||||||
|
Within the limitations of the experimental methods employed in the present study, the following conclusions can be drawn: All tested materials have antibacterial properties against the tested bacteria, and experimental calcium based cement exhibited remarkable antibacterial activity against tested bacteria, comparable to that of polycarboxylate cement and better than biodentine. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Emad F. Alkhalidi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Talal H. Alsalman – Analysis and interpretation of data, Drafting the article, Final approval of the version to be published Amer A. Taqa – Substantial contributions to conception and design, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Emad F. Alkhalidi et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||